Did you know that nearly 80% of FDA warning letters (Form 483) issued in the last five years were due to data integrity issues, with a significant portion related to digitally generated records? The 21 CFR Part 11 rule deals with electronic records and signatures. IA solid compliance checklist ensures you meet FDA standards. A compliance checklist helps you avoid risks, ensuring your electronic records are secure and reliable.
In this post, we'll explore 21 CFR Part 11 and offer tips to keep you compliant.
What is 21 CFR Part 11 Compliance?
For any company handling electronic records and signatures, knowing about 21 CFR Part 11 compliance is key. This FDA rule considers electronic records equal to paper ones and ensures that all digital documents are safe, reliable, and trustworthy.
Basics of 21 CFR Part 11
21 CFR Part 11 was introduced in March 1997. It sets rules for using electronic signatures and records in FDA-regulated areas. It ensures the information is real and secure, with clear trails and system checks.
Following these rules helps protect important data, builds trust in digital records, and meets the FDA's high standards.
Why is 21 CFR Part 11 Compliance Important
For companies in regulated fields like pharmaceuticals and medical devices, following 21 CFR Part 11 is key. It ensures that electronic records and signatures are reliable. This rule helps maintain a high quality and safety standard in your products and services.
Not following this rule can lead to big problems. You might face fines, product recalls, or even have to shut down. The harm goes beyond money; it can hurt your reputation and how well your business runs. Staying compliant with 21 CFR Part 11 protects your company's image and keeps your customers and stakeholders trusting you.
21 CFR Part 11 Noncompliance
Not following 21 CFR Part 11 can lead to big problems for your company. FDA inspectors might find issues during their visits, which could result in a Form 483 report showing the problems they found.
This first warning is a sign of bigger issues to come. If you don't fix these problems, you could face even worse consequences, including warning letters or product recalls.
Ignoring these rules can hurt your business in many ways. It can damage your reputation and make it hard to operate. Knowing the dangers of not following the rules helps companies stay on track.
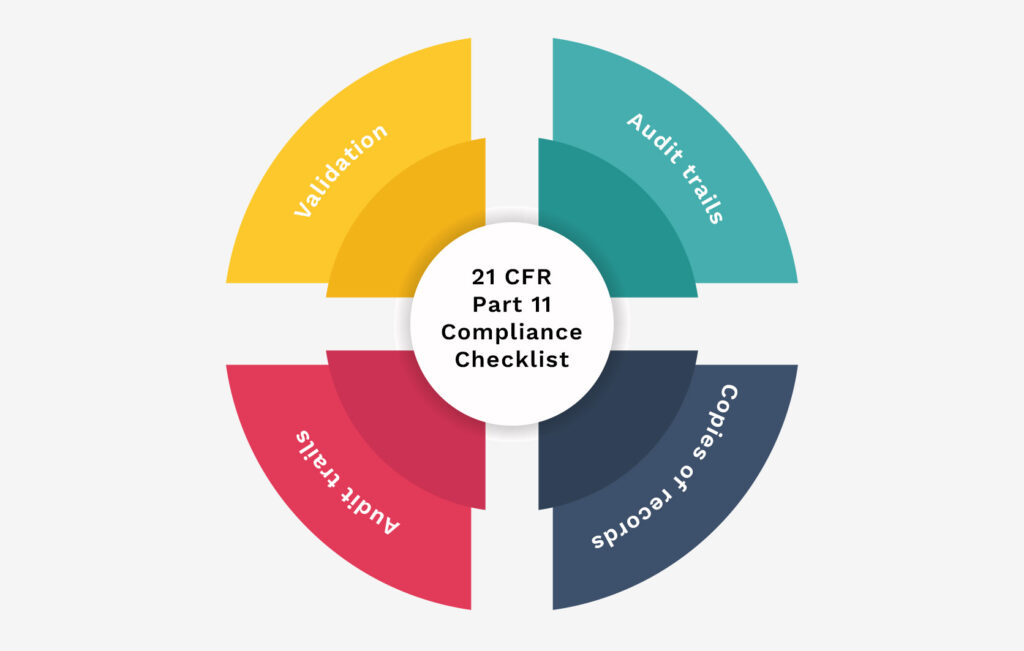
To meet 21 CFR Part 11 standards, you must pay close attention to detail and check many parts of a detailed checklist. Focusing on these areas ensures your data is safe and follows the rules.
Part 1: Validation
Make sure that systems work as they should. You must test software and processes well to confirm that they work correctly. This step helps reduce risks with electronic records and signatures.
Part 2: Audit trails
Audit trails show all system actions. Check these logs often to see any record changes. This ensures your operations are transparent and accountable.
Part 3: Copies of records
Keeping accurate copies of records is essential. You should store both electronic and paper records well. This makes it easy to find and review them later. This practice also helps your operations run smoothly.
Part 4: Record retention
Record retention must follow 21 CFR Part 11 rules. Having a clear plan for how long to keep records helps prevent data loss and keeps you in line with the law. Checking your retention policies often ensures you stay compliant.
Maintaining 21 CFR Part 11 Compliance
Your team needs a proactive plan to comply with 21 CFR Part 11. Regularly check and update your systems and processes. Staying current with new regulations also helps you stay compliant.
Employee training is a big part of this. Regular training sessions help keep important topics like compliance and electronic signatures fresh in everyone's mind.
Regular audits are also important as they help you see if you follow the rules. They also help find problems early so you can fix them before they worsen. Making sure you're well-prepared for audits helps keep your compliance efforts strong.
How CFR Part 11 Compliance Software Can Help
Using CFR Part 11 compliance software, which is made for industries with strict rules, makes it easier to follow the rules. It also helps create audit trails, making tracking changes and finding records easy.
An electronic quality management system keeps records safe and ready for FDA checks. This means your team can answer questions quickly, reducing lost time.
Good record management software lowers the risk of not following rules. It reduces manual work, saves time, and reduces mistakes. With changing rules, having the right software is very important.
Develop 21 CFR Part 11-Ready Software with Kohezion
Kohezion helps your team create 21 CFR Part 11-ready software that fits your needs. This custom compliance solution improves your workflow and keeps you in line with standards.
Kohezion's tools, like access control and validation, help keep your records safe and secure, ensuring your software meets all the necessary rules.
Working with Kohezion lets you build workflows supporting compliance and quality management. Customizing your software to meet these standards helps you work better and avoid risks.
Conclusion
Getting 21 CFR Part 11 compliance is key for companies in regulated fields. It's especially important for managing electronic records and signatures. A detailed checklist can help you handle the complex rules well.
This approach meets industry standards and protects your business from legal problems. It keeps your operations in line with the rules.
It's also important to watch for any rule changes. Strong software can make meeting 21 CFR Part 11 needs easier. This focus on quality and accountability improves your data's integrity.
Being dedicated to 21 CFR Part 11 compliance is a big step for your company. It shows you're serious about quality and following FDA rules. This commitment helps keep your business strong and respected.
Contact us today for more information on how Kohezion can help you achieve 21 CFR Part 11 compliance. We can provide the tools and support you need to manage your electronic records and signatures effectively.
Start building with a free account
Frequently Asked Questions
A 21 CFR Part 11 compliance audit should be conducted regularly to ensure ongoing adherence to the regulations. Audits should be performed annually or whenever significant changes are made to electronic systems or processes. Regular audits help identify and address any compliance issues before they become major problems.
Following a structured validation process helps organizations ensure their electronic systems are validated for compliance. This includes documenting validation protocols, performing system tests, and ensuring the system functions as intended under normal operating conditions. Regular reviews and updates of validation documents help maintain compliance.
An electronic record audit trail should include detailed information about all changes made to the records, including who made the changes when they were made, and what was changed. It should be secure, tamper-evident, and easily accessible for review. The audit trail must be maintained for a defined period and can track all relevant actions to ensure data integrity and compliance.